Enzymes are nature’s own working bees, and the sole reason chemical reactions can be carried out in the neutral environment within all cells.
Recently, chemists have tried to harness the great potential of enzymes in reactions, which are difficult to achieve by common chemical methods. One of the most attractive enzyme classes is oxidoreductases, more specifically oxidases, which use molecular oxygen either as an oxidant or as an electron acceptor for oxidation.
Oxidases – Advantages and Limitations
Compared to common chemical methods, enzymatic oxidation has many advantages, the main one being conversion at ambient temperatures and pressures. However, as enzymatic oxidation is done in aqueous media, the overall conversion is highly limited by solubility of oxygen in water, and by extension, the oxygen mass transfer rate through the gas-liquid interface.
Download pdf
Case Study # 10, rev 2, 15 Aug 2023,
By Sanjith Krishna, Julie Østerby Madsen and John M. Woodley,
Dept. of Chemical and Biochemical Engineering, The Technical University of Denmark
Hence, before oxidases can be considered relevant for larger scale oxidations, the mass transfer rate must be optimised to meet the enzymes oxygen demands and increase the volumetric productivity. One way to do this is to optimise the reactor itself, an example of such a reactor is the Scalable Agitated Baffle Reactor (SABRe) from Stoli Chem.
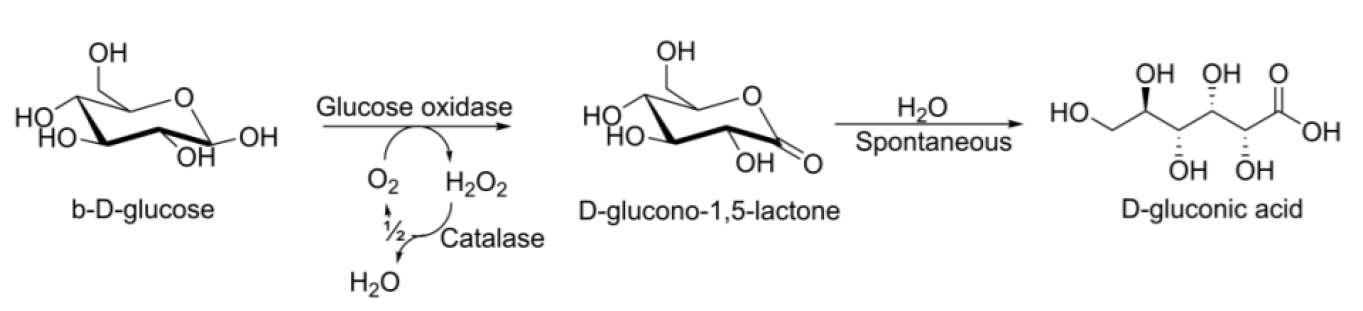
The SABRe is a novel multistage flow reactor with small stirred-tanks in series, which promises high gas-liquid mass transfer rates. The aim of this project was to study the capabilities of the SABRe reactor in an enzymatic oxidation setting, by means of a model reaction with glucose oxidase (EC 1.1.3.4).
In the model reaction, glucose was converted to glucono-1,5-lactone by means of glucose oxidase and molecular oxygen. The product was subsequently converted to gluconic acid by spontaneous hydrolysis. The reaction was repeated under the same conditions in both a common bench-scale batch reactor and in the SABRe.
Experimental Procedure
The experiments were run in 400mM phosphate buffer solution at pH 7 and 500 rpm with 100mM glucose and 0.27 mM oxygen as substrates and 2g/L glucose oxidase (Novozym® 28166 from Aspergillus niger). Moreover, 0.1g/L catalase was used to convert the resulting hydrogen peroxide to water and oxygen to avoid product inhibition of the glucose oxidase.
For the batch reaction, a 250mL reactor with 100mL working volume was used, corresponding to the 100mL reactor insert used in the SABRe. Samples were taken from the batch reaction from 10-150min reaction time, while the flow in the SABRe were varied to obtain similar results based on residence time in the reactor.
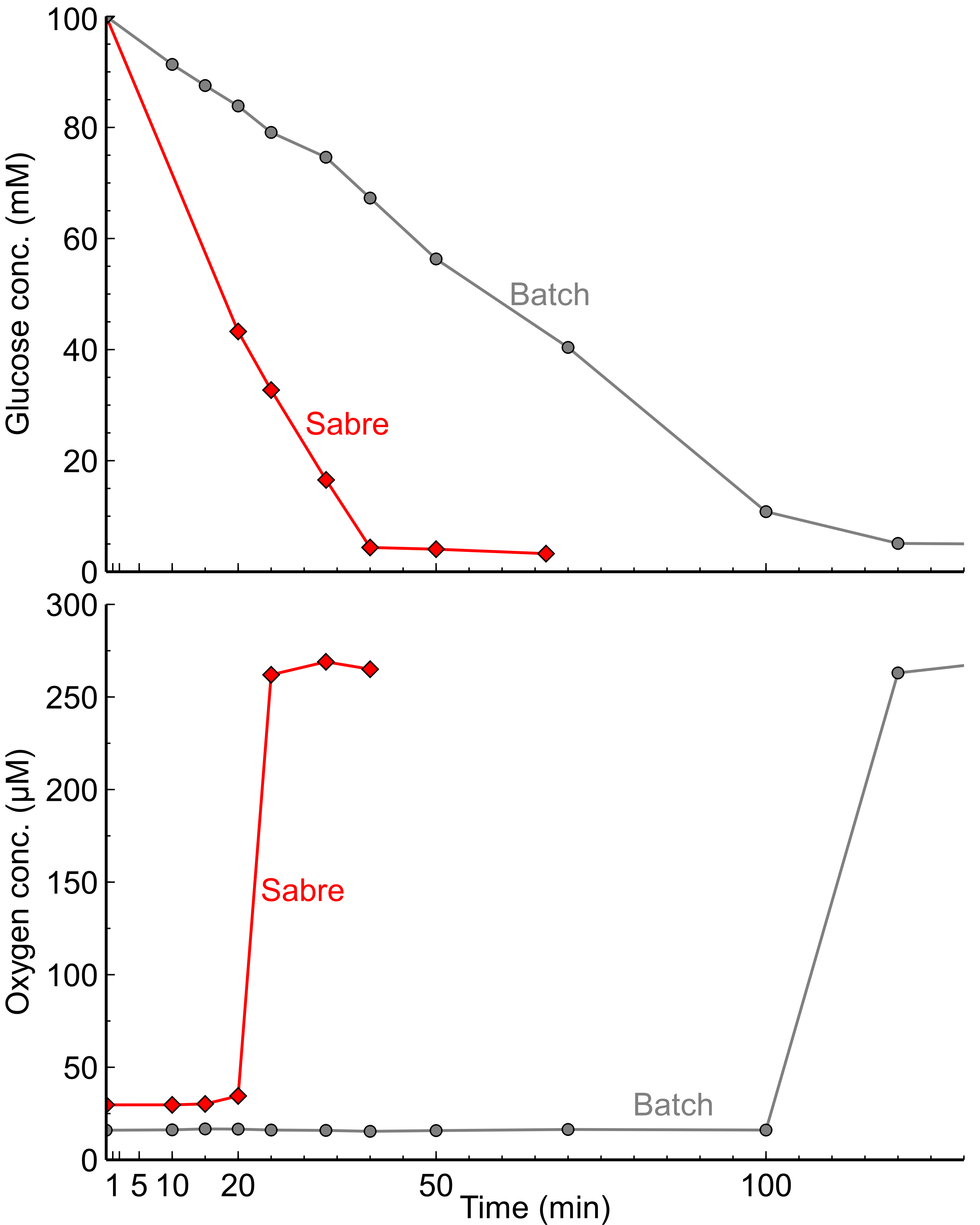
Results
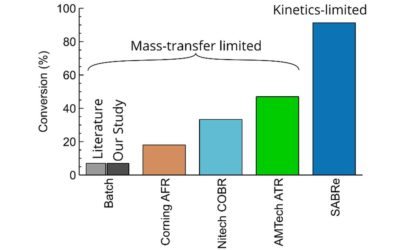
Compared with the batch reactor, it is clear that the reaction rate of the model reaction is much higher in the SABRe, given the reaction is completed almost three times faster.
The dissolved oxygen values were slightly higher in the SABRe during the reaction, which could indicate a superior oxygen transfer. This could be due to the greater gas-liquid interfacial area caused by the multiple impellers (10) in the SABRe as compared to the two impellers in the batch.
Overall, based on these results it could be deduced, that the SABRe has great potential for oxygen dependant enzymatic reactions, in which the solubility of oxygen in liquid is a limiting factor.
The SABRe system (available in steel, Hastelloy or glass) is suitable for a wide range of chemical applications. Combining simplicity with superb reaction control, SABRe is the best choice for simple, safe and cost effective chemistry.
What can the SABRe do for you today? Get in touch and arrange a trial.
Other SABRe case studies:
Steven’s oxidation with Vapourtec
1.4 kg/day multiphase oxidation obtained integrating SABRe system with Vapourtec’s R-Series
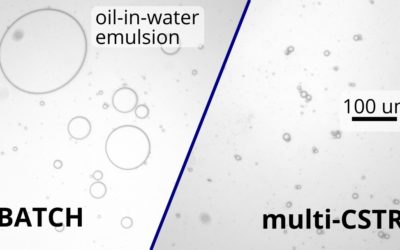
Consistent oil-in-water emulsions in continuous flow
Using a continuous multi-CSTR system allowed us to make droplets 2.5 times more uniform compared to a batch reactor
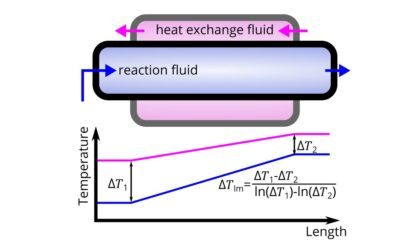
How to calculate heat transfer in continuous flow applications
Continuous flow (such as micro-reactors) are superior for exothermic reactions. How do you compute the thermal performance of a reactor?
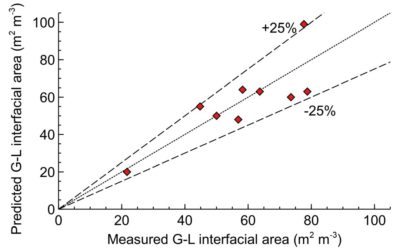
Maximising interfacial gas-liquid area with Scalable Agitated Baffle Reactor (SABRe)
How the SABRE system provides large gas-liquid area to maximise the reaction throughput and selectivity.
Comparison of continuous reactors in enzymatic esterification
We showed superior performance of SABRe in the enzymatic (liquid-liquid) esterification.
How residence time affects product quality in flow chemistry
How residence time is vital for throughput and product quality.