Formulation chemistry stretches from flavours and fragrances, to food and beverages, to agrichemicals, to cosmetics. Although no chemical bonds are made or broken, formulation is a diverse field utilised across products we find in everyday life. One aspect of formulation chemistry which is of particular interest in foods, beverages and cosmetics is that of emulsions. They can be found in a wide variety of products, from mayonnaise to face cream, where quality is determined by both the size of the bubble, and its stability in solution.
Download pdf
Case Study # 07, rev 3,
2 Nov 2021, By Samuel Adams
Emulsions occur where two immiscible liquids are mixed to form small bubbles or droplets. In a majority of cases, a third agent, a surfactant, is added to separate two phases and stabilise large interfacial surface area.
Emulsions, therefore, are determined by
- Chemistry of the interacting phases
- Properties and amount of the surfactant
- Quality and type of mixing that puts these components together
The Experiment: batch vs flow comparison
To determine how Scalable Agitated Baffle Reactor (SABRe) performs against batch in liquid-liquid emulsion forming, we performed a comparative study under identical conditions. A 3% commercial surfactant (sodium laureth sulphate) solution was fed into the reactor at a 20:1 volumetric ratio with oleic acid at a residence time of 20 minutes. In the comparative batch experiment, 100 mL of 3% commercial detergent solution and 5 mL oleic acid was stirred for 20 minutes. Both stirring rates were 1200 rpm. The resulting emulsions were sampled and analysed by optical microscopy.
The results
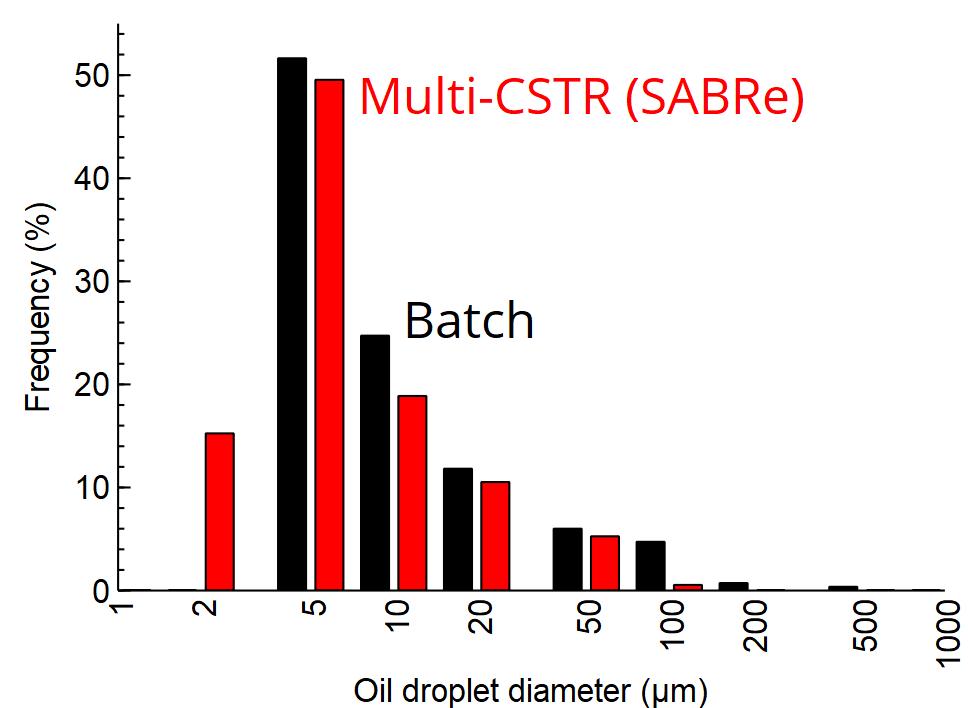
The opposite image shows emulsions formed by SABRe (left) compared with batch (right). From multiple images, the size distributions of the particles were calculated. From the images, it can be clearly seen that smaller micelles are formed in SABRe than in batch, with the distribution graph also showing an increase in size in batch. The mean particle diameter was found to be 2 times larger, with a distribution almost 8-fold bigger as determined by variance.

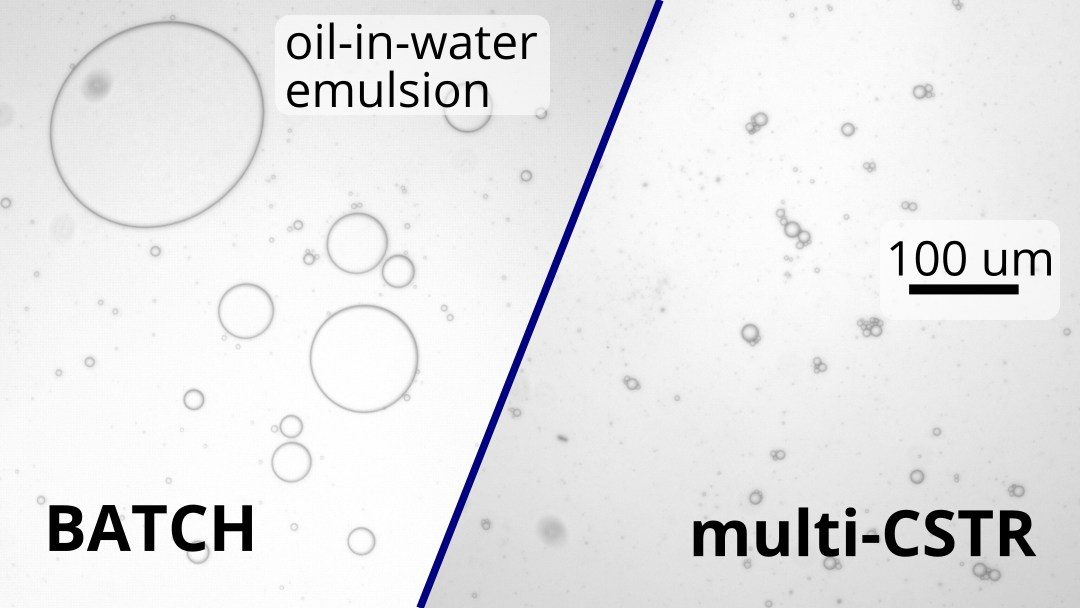
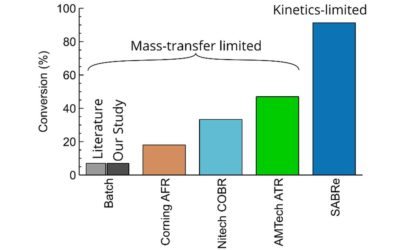
The results show the superior performance of SABRe’s multichambered reactor design compared with batch. With an impeller in every chamber combined with smaller volumes per chamber, mixing power is greatly improved, translating to smaller micelles and a better quality emulsion.
Thanks to continuous processing, higher throughputs can also be achieved in a smaller plant footprint!
The SABRe system provides the highest performance in class in interfacial mixing for more efficient, faster, and precise chemical production.
The SABRe system (available in steel, Hastelloy or glass) is suitable for a wide range of chemical applications. Combining simplicity with superb reaction control, SABRe is the best choice for simple, safe and cost effective chemistry.
What can the SABRe do for you today? Get in touch and arrange a trial.
Other SABRe case studies:
Improvement of enzymatic oxidation in the continuous Scalable Agitated Baffle Reactor (SABRe) system
Case study on enzymatic oxidation by Prof John Woodley
Steven’s oxidation with Vapourtec
1.4 kg/day multiphase oxidation obtained integrating SABRe system with Vapourtec’s R-Series
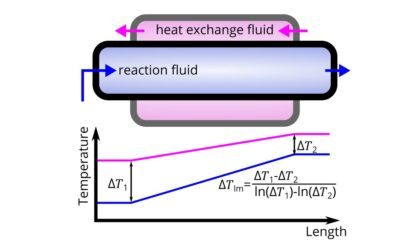
How to calculate heat transfer in continuous flow applications
Continuous flow (such as micro-reactors) are superior for exothermic reactions. How do you compute the thermal performance of a reactor?
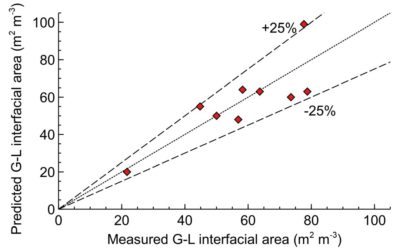
Maximising interfacial gas-liquid area with Scalable Agitated Baffle Reactor (SABRe)
How the SABRE system provides large gas-liquid area to maximise the reaction throughput and selectivity.
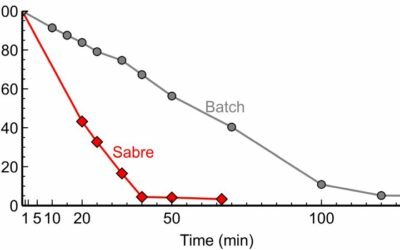
Comparison of continuous reactors in enzymatic esterification
We showed superior performance of SABRe in the enzymatic (liquid-liquid) esterification.
How residence time affects product quality in flow chemistry
How residence time is vital for throughput and product quality.