The real advantages of continuous flow chemistry
What is flow chemistry and what it means for a real-life chemist – that is what we discuss here.
“Flow chemistry surges forward” states the headline of the influential Chemistry World publication and explains that “flow processes can produce higher yields, and be safer, cleaner and cheaper to set up and operate”.
Contents
What is flow chemistry? Batch vs flow
Utilisation and efficiency in flow chemistry
Real-life applications of flow chemistry
Fundamental benefits of continuous processing compared to batch
Commercial benefits of flow chemistry
Why does batch still dominate chemical production?
When is it worth considering a continuous process?
Where does flow chemistry have a decisive lead over batch?
Updated on 27 Jan 2022, by Dr Nikolay Cherkasov
About the author:

Nikolay is an experienced researcher having 50+ peer-reviewed scientific publications and 10 years experience in continuous processes. He is a member of IchemE.
What is flow chemistry? Batch vs flow
A conventional way of doing chemistry is batch. The batch reactor is simply a vessel with a volume of microliters to cubic meters filled with chemicals that are subjected to mixing, temperature and pressure. A batch synthesis is repeated many times over to produce more material. Chemistry often starts in 1-100 mL round-bottom flasks (batch reactors) and travels through several steps into scale-up, pilot, and production at 1-10 m3 reactors.
In flow chemistry, we move materials constantly through the vessel. Like in batch, the continuous reactor vessel contains the materials and provides sufficient time for the reaction. The major difference is that we do not have to start and stop the process to produce more. We can scale-up flow processes by using wider reactors, longer reactors, put multiple reactors in parallel, or combine these approaches.
Therefore, one major difference between the batch and flow reactors is that flow processes can be scaled up by running for longer without incurring additional costs such as repeated cleaning, cooling and heating which required in batch. Therefore, a flow process is intrinsically more efficient and has a substantially lower downtime.

Utilisation and efficiency in flow chemistry
Industry experts we surveyed indicate that a “fully utilised batch reactor” is used only about 30% of the time for chemistry in a “good manufacturing practice” (GMP) environment. In the fine and bulk chemicals industries, utilisation may be higher but still intrinsically limited – the rest of the time the reactors are cleaned, heated, cooled… Larger reactors require much longer to heat up or cool down.
Continuous processes, on the other hand, do not require such downtime. It does not mean that any flow process is 100% efficient – depending on the process and industry, the downtime varies. The key is that continuous processing theoretically allows achieving 100% reactor operation and has demonstrated utilisation of above 90% with only annual maintenance and inspection downtime.
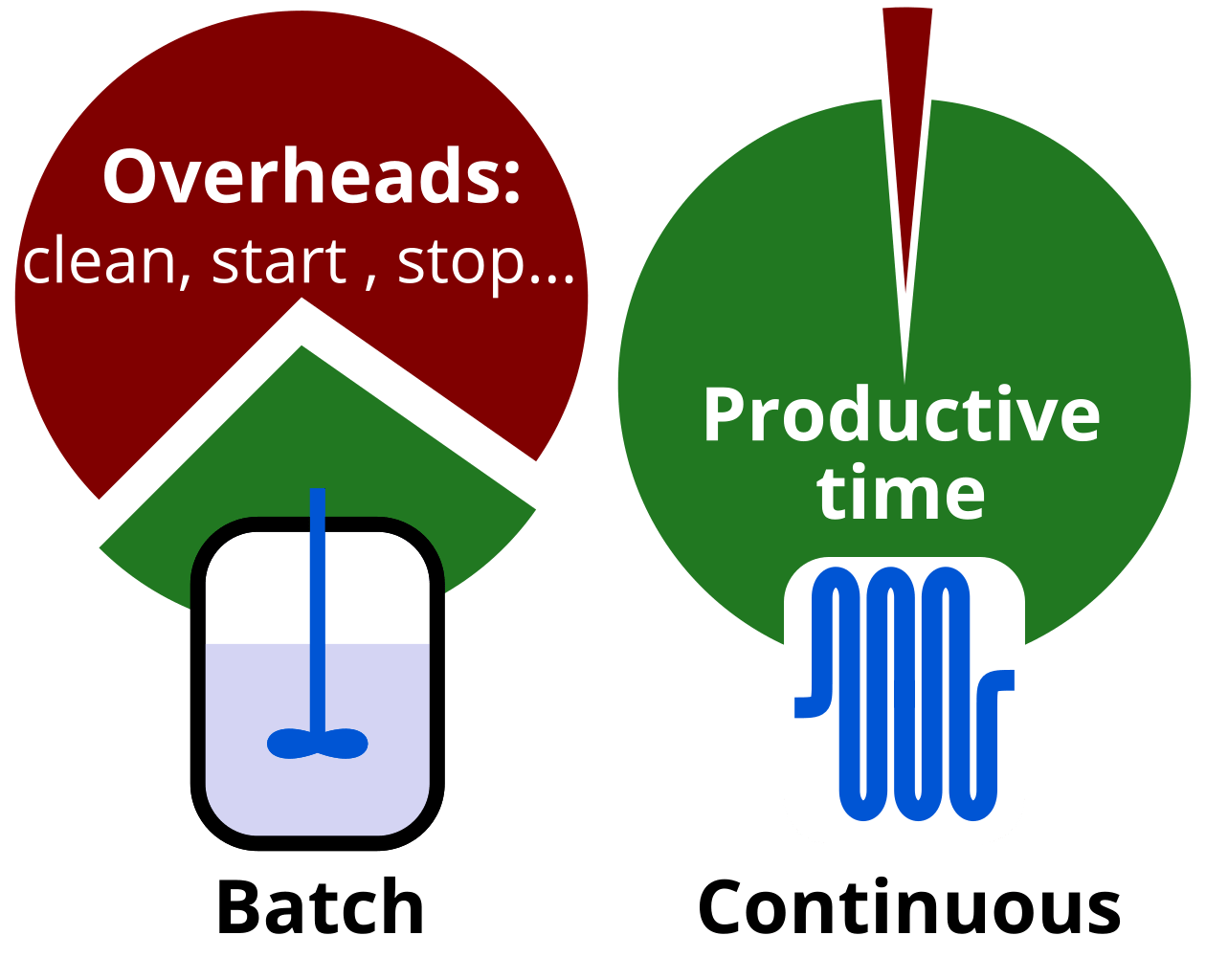
Utilization of batch and flow reactors
Real-life applications of flow chemistry
Flow chemistry (or continuous flow chemistry) is not novel. All top 300 commodity chemicals by volume are produced in continuous plants. [reference and visual for the fine chemicals book]
At the turn of the millennium however, the term “continuous flow chemistry” attracted increasing attention in fine chemicals and pharmaceuticals – areas dominated almost exclusively by batch manufacturing.
All large-volume processes use continuous due to the discussed efficiency benefits. At a smaller scale (small, we are still talking about 10,000 ton a year), the batch reactors dominate because setting up a continuous process is expensive. Traditionally, establishing a continuous process requires a substantial upfront investment that is not justified when the production scale is small.
Yet, the situation is changing because continuous flow reactors can be multifunctional, scalable and rapidly translated from batch.
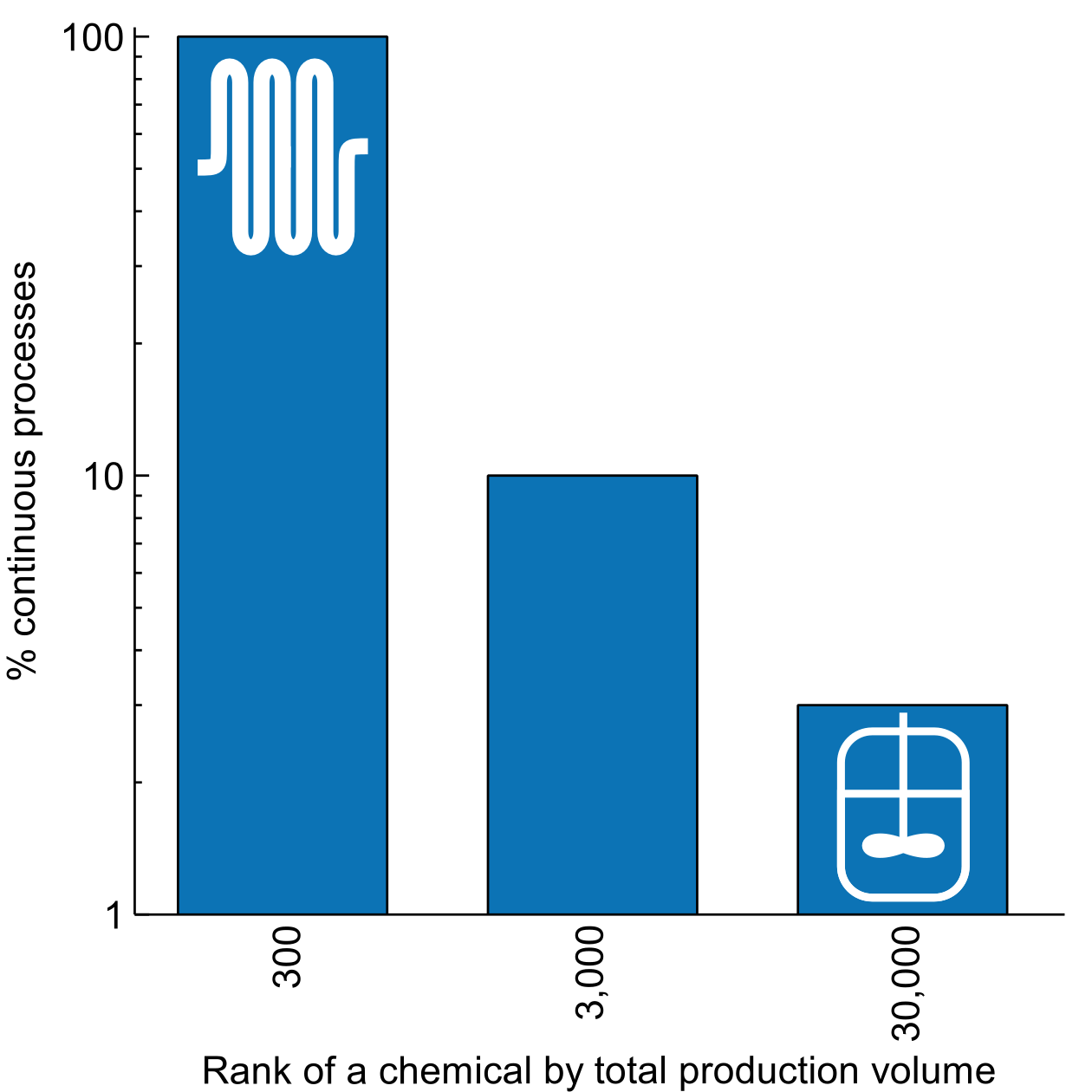
Proportion of continuous processes depending on the scale of a chemical production volume
Fundamental benefits of continuous processing compared to batch
The differences between batch and flow are exciting not only for accountants. There are strong fundamental reasons why flow chemistry brings an edge compared to batch:
Surface to volume ratio
The reactor external surface to volume ratio determines the rate of heat transfer. This, in turn, opens a way to perform exothermic reactions and tightly maintain the reaction heat.
These advantages are often vividly demonstrated in micro-reactors and milli-reactors – continuous flow reactors with the channel diameters below 1mm. For example, a 100 micrometre tube has a surface to volume ratio 500 times larger than a 100 mL round bottomed flask.
| Reactor | Surface to volume ratio (m2 m-3) |
| 10 mL round bottom flask | 200 |
| 100 mL round bottom flask | 80 |
| 0.1mm (internal diameter) tube | 40,000 |
| 2mm (internal diameter) tube | 2,000 |
Rapid mixing
Rapid mixing increases reaction rates and may reduce over-reaction (decrease the impurity levels and/or increase the selectivity).
When we have small reaction channels, the main mixing mechanism moves from convection (movement of fluid) to diffusion. Diffusion, however, is extremely slow when applied to macroscopic distances of centimetres. Diffusion alone is irrelevant in batch, that is why we have agitation. In small channels, diffusion mixing is exceptionally quick.
In a continuous reactor with small channels, the whole reaction is mixed in a matter of milli- and micro-seconds.
Broader process windows
Reaction conditions in batch are severely constrained by the possible conditions. A majority of batch reactors have a narrow process conditions window they could operate in. For example, a batch typical reactor has a rated (maximum) pressure of below 5 bar, and temperature – below 150 oC.
Not surprisingly, most batch chemistries take place at the temperature range of -20 – 150 oC, and a pressure below 5 bar. These conditions do not mean that the chemistry is optimal, or even the costs are minimised.
Another problem of going beyond the traditional process conditions is safety. A large-scale process requires a detailed Hazard and operability (HAZOP) study. It takes time money and more “adventurous” process conditions may require a lot of consideration, mitigation… A financial gain in the product costs may not be justified by the upfront safety costs, development delays and the corresponding uncertainty.
Smaller continuous reactors provide faster heat transfer and could withstand much higher pressure (20-200 bar). The plot shows that a tin foil of 0.5mm stainless could withstand 100 bar pressure in a reactor 2mm outer diameter, while a 10 cm diameter for the same pressure requires almost 1 cm of metal wall.
There are high-pressure batch reactors but they are often bespoke units with large capital cost implications. A smaller continuous reactor may produce as much as batch, but it may require simpler safety assessment and contain much harsher process conditions due to its smaller volume. A reaction that requires hours in batch could be performed at a higher temperature in flow within minutes.
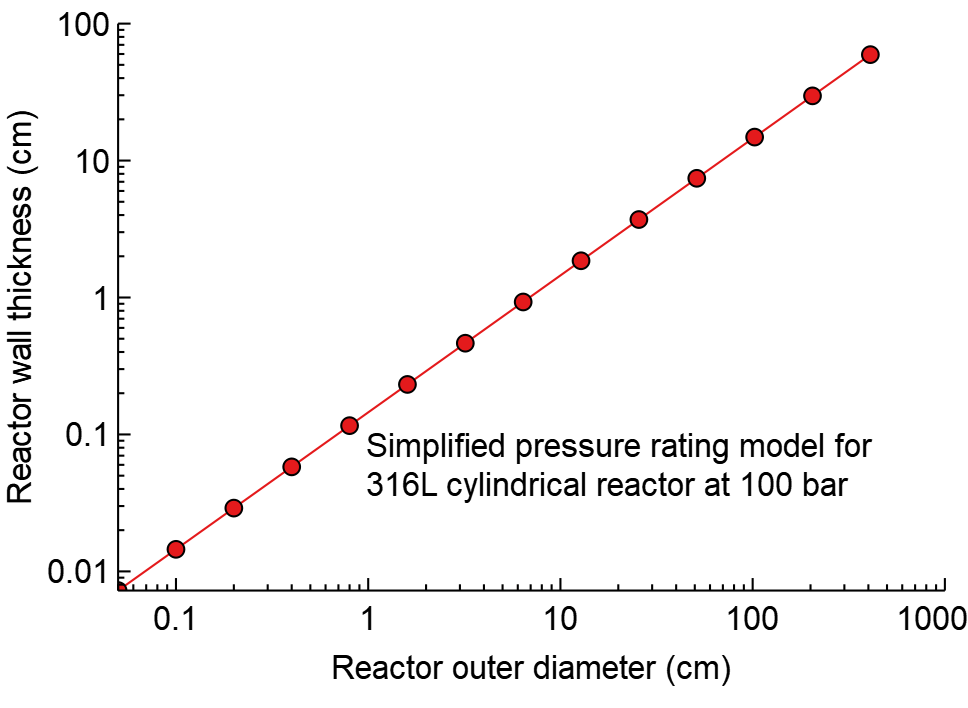
Idealised reactor wall thickness for a 100 bar reactor. Wall thickness grows hugely with the reactor diameter.
Commercial benefits of flow chemistry
Flow chemistry can bring significant benefits. The reactions could be performed faster, safer, with rapid scalability and higher quality. There are 3 main drivers to use flow chemistry:
- Safer – small reactor volume
The case of safety does not imply that batch production is not safe. The continuous processes are simply much more intrinsically safer. Lower risks mean less capital into safety measures and a broader scope for chemistry to reduce the number of steps and maximise the yield.
Flow processes are usually considered intrinsically safer because of a dramatically lower reactor volume in flow reactors compared to batch. 1 mg of an explosive azide is far less hazardous than 1 gram.
In batch chemistries, a hazardous intermediate is obtained first and then reacted. Depending on the reactor volume, this means a hazardous inventory of kilograms to tons – a major hazard. This does not mean that once a year a batch reactor explodes; such hazardous batch processes are not run at all regardless of any benefits the hazardous chemistry could bring.
- Scalability
Microreactors with their small volume do eliminate bulky agitators and accelerate heat and mass transfer. As such, the specific reaction rates could be significantly increased per unit of reactor volume. There are strong cases where a factor of 1,000X improvement could be realised in continuous flow.
At a smaller scale – laboratory and kilo-plant – the continuous synthesis brings the possibility to develop a process and run them for several days or weeks to obtain the required quantities. While the pumps and basic process control work, people do not have to operate (as in batch) – the continuous processes reactors saving on additional scale-up R&D.
- Increased quality
Rapid heat and mass transfer in continuous processes bring much more precise process control. As a result, the possibilities of a reaction runaway and the corresponding side-reactions are greatly reduced. With less by-products, precise and consistent reaction timing, the amounts of impurities go down.
Most importantly, such an increased product quality is significantly more consistent over time because continuous processes could accommodate minor process disturbances better. A minor momentary excess of one of the components may result in a local hot spot, but this energy will be dissipated quickly due to excellent micro-mixing and thermal performance of continuous processes.
Why does batch still dominate chemical production?
Batch processes are still the main way of producing chemicals. Not every process is worth converting into flow, not every process will benefit. The major advantages of batch processes include:
Low-cost multipurpose plants. Batch reactors are versatile – a vast majority of chemistry could be performed in batch. A single investment into a plant could produce almost any material.
Availability. Batch plants of any scale are readily available and are mostly inter-exchangeable. You could contract a laboratory study in a 50 mL reactor, or a production in 10 m³ reactor – the reactor technology or availability will unlikely be your limiting factor.
Predictability. Batch processes are known for centuries. Process chemists know what to expect, know possible problems, and how to solve them. Problems do occur, but these are not out-of-the-blue hurdles but mostly minor process deviations.
Scalability. Process chemists know how to scale up a batch process following a well-trodden path of laboratory, pilot, and manufacturing stages. Each stage helps to reduce uncertainties and improve the procedures. Yet, the path is known and success is almost inevitable.
When is it worth considering a continuous process?
Roberge and co-workers from Lonza indicated that about 50% of processes would benefit from continuous processing:
- Rapid reactions completed within seconds. Such reactions require rapid mixing. The reactions involve reactive species such as chlorine, bromide, amines, acid chlorides, and organometallic compounds (lithium and Grignard-type chemistry).
- Reactions completed within 1s to 10 minutes. Being relatively slow reactions, they are often controlled by intrinsic reaction rates and not by the mixing performance; but they benefit from substantial heat transfer performance improvements resulting in higher selectivity and product quality.
- Slow reactions that require more than 10 minutes, but where rapid mixing and heat transfer brings safety and quality advantages. Examples include reactions that require relatively high temperature and pressure that is often unavailable in batch.
Plutschack et al. write points the following factors as decisive in selecting flow over batch:
- Improved safety
- Rapid reactions
- Reactions that are adversely affected by temperature
- Photochemistry and electrochemistry
- Multiphase systems (gas-liquid and solid-liquid) as they greatly benefit from rapid mass transfer
- Emulsions where scaling up could be difficult

Where does flow chemistry have a decisive lead over batch?
Making efficient high-energy chemistries feasible. Rapid heat and mass transfer is crucial in opening chemistries that are impossible in batch. Lithiation is one such example requiring cryogenics in batch (-80 C) to maintain reaction rates and avoid thermal runaway that ruins the product quality by favouring side-reactions.
Reaction telescoping. Not all processes are optimal under the same conditions. Stitt notes that “the advantages of multifunctionality are rapidly lost if design or operation is not proximate to the optimal requirements for the various integrated co-processes” [Stitt]. Yet, there are plenty of examples where optimal parameters for one process are very broad. For example, quenching could open new reaction possibilities. You could handle hazardous reaction intermediates and obtain kilograms and tonnes with only grams of hazardous compounds that are constantly produced and used up.
Maximising efficiency. “If the campaigns are short, cleaning can be the biggest «product» in a multi-purpose plant, Rolf Dach (Boehringer-Ingelheim)”[fine chemicals]. In larger batch campaigns, changeover times could be lower – only 50%. This means that you pay for 100% time and premises, but use the reactor to obtain products only 50% time; often only 30%. Flow reactors minimise or eliminate changeover, so the benefits of flow increase dramatically with production scale.
Could we combine the benefits of batch and flow?
The advantages of batch and flow are often mutually exclusive. Flow reactors are available in multiple types; they do limited tasks exceptionally well, but a multipurpose flow plant becomes very expensive due to purchasing lots of specialised equipment.
There is no general way to combine all the benefits, but we could combine the majority of benefits. And the core is as old as batch – a continuously stirred tank reactor (also sometimes called semi-batch) – a system where the reactants are constantly added and the products withdrawn.
They are multipurpose, predictable in behaviour and scale-up. A series of stirred tanks enables efficient telescoping and maximises efficiency. Small stirred tanks also have high heat and mass transfer rates. However, many stirred tanks in series are required to control residence time well. If you have a selective process, you may need 50 or 100 effective stirred tanks to ensure materials spend precisely the required time in a reactor. A series of many stirred tanks is cumbersome, expensive and complex.
Are series of stirred tanks solution to all problems?
No, series of stirred tanks is not an ultimate solution. There are plenty of cases where batch or a microreactor is the best tool. Batch is greatly beneficial in slow processes which could not be intensified. An intrinsically slow process does not benefit much from elimination of the (infrequent) start-stop routine.
Conventional microreactors also have strong merits – exceptionally fast chemistries do greatly benefit. Some chemistries are simply impossible otherwise – see flash chemistry. These processes require fractions of a second time; they could be scaled up to kilos in a single microreactor. Yet, there is a substantial area in between where continuous flow chemistry may bring benefits.
(infographic? – reaction time. Too fast – flow; too slow – batch; middle – flow/batch/cstr)
Faster, cheaper, and safer chemistry with series of stirred tanks
Our novel SABRe system contains 10+ stirred tanks in series in a single package – a single pressure vessel with shaft and magnetic drive, and without numerous leak-prone connections.
Yet, with excellent control of reactions and residence time. Hydrodynamics of batch processes all apply – you may reasonably predict the behaviour and scale-up the approach. You do need to do piloting, but you have high confidence upfront being able to use known engineering correlations to heat and mass transfer. Rapid telescoping is possible by adding several reaction components to open new chemistry.
How to start – Reading material
There are several classical papers that provide in-depth analysis of continuous flow chemistry from various angles:
(1) Plutschack, M. B.; Pieber, B.; Gilmore, K.; Seeberger, P. H. The Hitchhiker’s Guide to Flow Chemistry. Chem. Rev. 2017, 117 (18), 11796–11893. https://doi.org/10.1021/acs.chemrev.7b00183.
(2) Jähnisch, K.; Hessel, V.; Löwe, H.; Baerns, M. Chemistry in Microstructured Reactors. Angew. Chemie Int. Ed. 2004, 43 (4), 406–446. https://doi.org/10.1002/anie.200300577.
(3) Hessel, V.; Kralisch, D.; Kockmann, N.; Noël, T.; Wang, Q. Novel Process Windows for Enabling, Accelerating, and Uplifting Flow Chemistry. ChemSusChem. 2013, pp 746–789. https://doi.org/10.1002/cssc.201200766.
(4) Jensen, K. F. Flow Chemistry—Microreaction Technology Comes of Age. AlChe 2017, 63 (3), 858–869. https://doi.org/10.1002/aic.15642.
(5) Dong, Z.; Wen, Z.; Zhao, F.; Kuhn, S.; Noël, T. Scale-up of Micro- and Milli-Reactors: An Overview of Strategies, Design Principles and Applications. Chem. Eng. Sci. X 2021, 10, 100097. https://doi.org/10.1016/j.cesx.2021.100097.
(6) Irfan, M.; Glasnov, T. N.; Kappe, C. O. Heterogeneous Catalytic Hydrogenation Reactions in Continuous-Flow Reactors. ChemSusChem 2011, 4 (3), 300–316. https://doi.org/10.1002/cssc.201000354.
(7) Newman, S. G.; Jensen, K. F. The Role of Flow in Green Chemistry and Engineering. Green Chem. 2013, 15 (6), 1456–1472. https://doi.org/10.1039/c3gc40374b.
We wrote this overview to give a quick start on the benefits of flow for Chemists and Chemical Engineers starting in the field.
Do you have any suggestions – please get in touch! We are eager to improve!


