Continuous flow chemistry brings advantages such as decreased inventory, reduced chemical manufacturing costs, enhanced throughput, and improved safety.
The precise process control enabled by continuous processing results in improved product quality.
Rapid heat transfer provides an opportunity to control temperature in rapid exothermic reactions. There are many benefits of efficient heat transfer, such as
- safer reactions as the risk of temperature runaway is reduced via rapid heat dissipation,
- precise reaction control with the ability to stop the reaction by stopping the reagent flow,
- improved reactor throughput allowing faster reagent addition.
Download pdf
Case Study # 05, rev 5,
2 Aug 2021, By Dr Nikolay Cherkasov
Calculating thermal performance – heat exchanger approximation
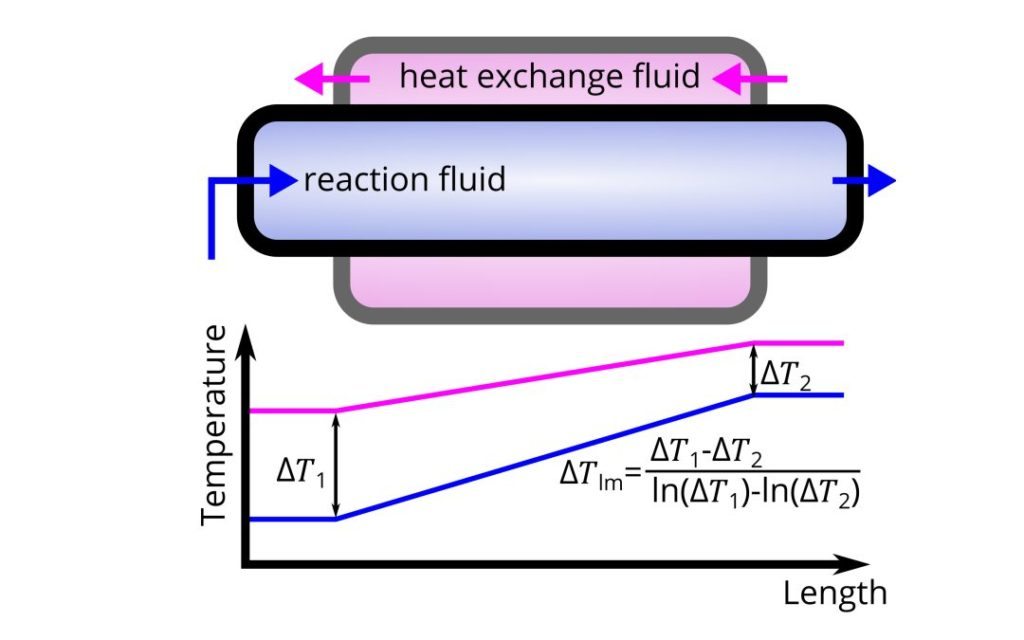
The basis for thermal calculations for a reactor is the same regardless of the reactor type. The principles apply to a batch reactor, a continuously stirred tank reactor, or a continuous flow reactor such as a plate or tubular reactor. The theory is simple and is based on a heat exchanger.
We have the process (or reaction) stream that contains chemicals, and an outer heat transfer fluid (or heat exchanger fluid). The heat flows via the reactor boundary (wall).
In some reactors, we use electric heating or some other heating mechanism, but the approach remains the same – heat travels via the reactor boundary to the reaction medium.
The driving force for the heat transfer is the temperature gradient along the reactor (ΔTlm). The higher the temperature difference between the heat exchange fluid and the process fluid at each point, the faster the heat transfer rate. Also, the heat transfer increases proportionally to the contact surface area (A).
The key parameter in heat transfer calculations is the overall heat transfer coefficient (U). It describes how quickly heat moves from the exchanger fluid, through the reactor boundary, and into the fluid bulk. The overall heat transfer coefficient depends on the properties of both fluids, on the reactor wall properties and geometry, and on the fluid velocities.
In the calculations, the heat gained by the process fluid (Qfluid) is equal to the heat lost by the exchanger fluid (Qexchanger); both are equal to the heat transferred (Qtransfer). Obviously, this neglects any thermal losses, which are insignificant at any sizable scale.
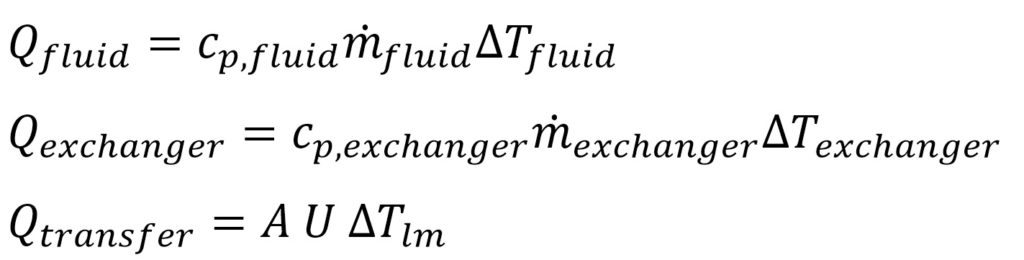
With these 3 equations, we could either
- calculate the overall heat transfer coefficient using known inlet and outlet process temperatures, heat capacities of the fluids (), and mass flow rates of fluids (); or
- calculate the outlet process temperatures of the fluid with the known reactor performance (U). This process is easiest to do by taking an estimate (guess) temperature and iterating until the system of equations is fulfilled.
In the SABRe system, we have extensively studied and optimised it for the highest heat transfer. Depending on the fluid velocities and properties, we observed the overall heat transfer coefficient of 700 – 1,500 W m-2 K-1 (depending on fluid and flow rates). For comparison, plate-based reactors have this coefficient of around 2,200 W m-2 K-1,[1] but plate reactors could barely handle slurries and any clogging results in writing off a $10-100k asset.
Managing the reaction heat
In case of strong exothermic reactions, we have the same heat exchanger with an additional heat coming from the chemical reaction (Qreaction):
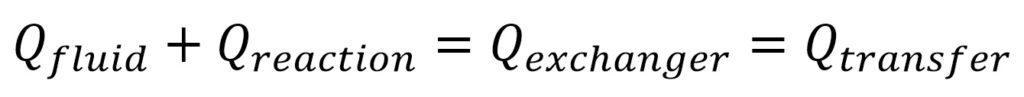
The reaction heat is well-known in most cases (the product of the reaction exotherm and the reactant molar flow rates).
The simplest calculation is the same. With the known heat transfer coefficient and process temperatures, you guess the outlet reaction and exchanger fluid temperatures and iterate until the equations are fulfilled.
In this model, we assume that the reaction fluid temperature changes uniformly along the reactor, meaning the reaction temperature only increases along the reactor rather than occurring at a hot spot. The hot spot situation is undesired from practical considerations as it decreases the process control because side-reactions are more likely in the hot spot.
With rapid mixing and heat transfer rates, Sabre enables handling exothermic reactions efficiently.

The SABRe system provides heat transfer performance comparable to that of micro-reactions in addition to higher flexibility, and ability to handle solids.
References: [1] E. Mielke, P. Plouffe, N. Koushik, M. Eyholzer, M. Gottsponer, N. Kockmann, A. Macchi, D.M. Roberge, Local and overall heat transfer of exothermic reactions in microreactor systems, React. Chem. Eng. 2 (2017) 763–775.
The SABRe system (available in steel, Hastelloy or glass) is suitable for a wide range of chemical applications. Combining simplicity with superb reaction control, SABRe is the best choice for simple, safe and cost effective chemistry.
What can the SABRe do for you today? Get in touch and arrange a trial.
Other SABRe case studies:
Improvement of enzymatic oxidation in the continuous Scalable Agitated Baffle Reactor (SABRe) system
Case study on enzymatic oxidation by Prof John Woodley
Steven’s oxidation with Vapourtec
1.4 kg/day multiphase oxidation obtained integrating SABRe system with Vapourtec’s R-Series
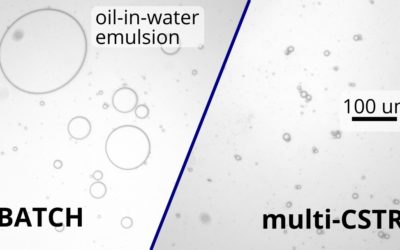
Consistent oil-in-water emulsions in continuous flow
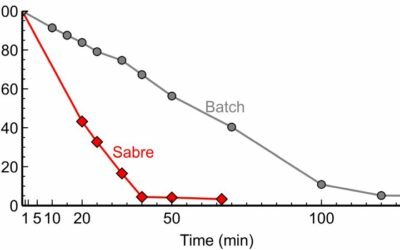
Using a continuous multi-CSTR system allowed us to make droplets 2.5 times more uniform compared to a batch reactor
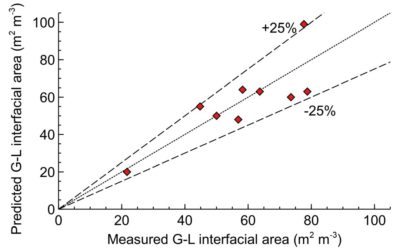
Maximising interfacial gas-liquid area with Scalable Agitated Baffle Reactor (SABRe)
How the SABRE system provides large gas-liquid area to maximise the reaction throughput and selectivity.
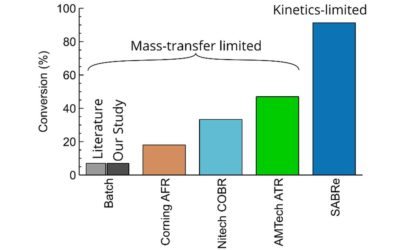
Comparison of continuous reactors in enzymatic esterification
We showed superior performance of SABRe in the enzymatic (liquid-liquid) esterification.
How residence time affects product quality in flow chemistry
How residence time is vital for throughput and product quality.