In this overview video, we discuss the pros and cons of a stirred batch reactor. Flexibility, scalability, reaction control, energy efficiency and catalyst lifetime are the parameters that are compared in our subjective view.
How does batch reactors work?
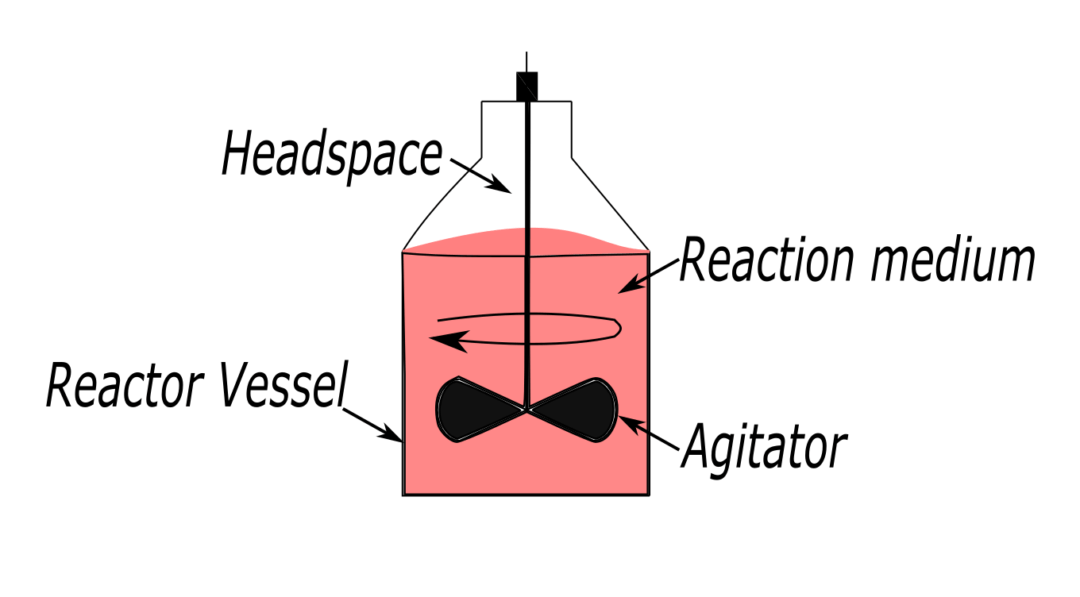
Batch, or stirred tank reactors are simply vessels that hold the reactants and allow them to mix. The batch reactor contains 4 main components.
1) The reactor vessel.
2) The reaction medium.
3) The headspace: an empty space above the medium. The headspace accommodates changes in the liquid volume.
4) The agitator: it is crucial as it allows the mixing of several components, and allows us to introduce or remove reaction heat.
Batch reactors are used from small round bottom flasks in the lab to thousands of litres in manufacture.
Reaction flexibility
Batch reactors are used in the manufacture of most chemicals as almost any reaction can be carried out in them. The versatility of this type of reactor means it can accommodate three different reaction phases: liquid-liquid, gas-liquid, and solid-liquid reactions.
Scalability
Batch reactors go from microliters to many cubic meters. Process scale-up is challenging. There is a lot of knowledge but it is not simply transferring the reaction from a smaller to a larger vessel.
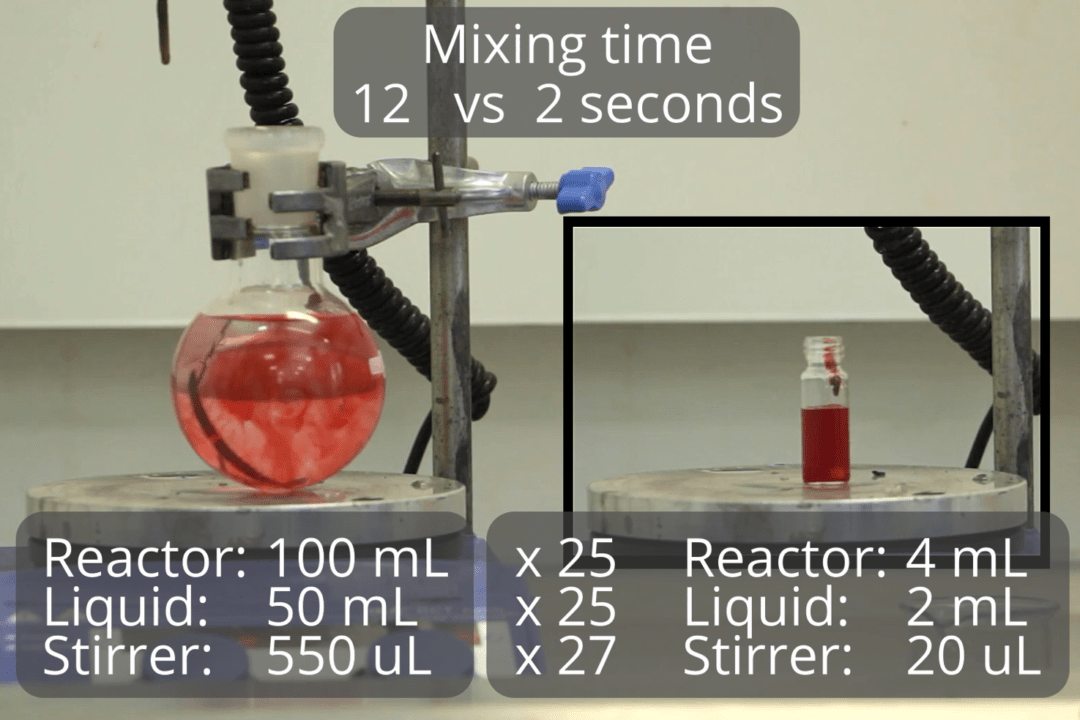
Reaction control
The key challenge of scalability is heat and mass transfer. The fluid velocity does not change proportionally to the reactor volume. In our demonstration, we added dye into a 4 mL vial – the colour became uniform in less than 2 seconds. When we add the dye into the larger flask, it took a 5-fold longer to mix. The flask was 25 times larger than a vial and it had a 25-fold larger stirrer. As a result – the much slower-moving fluid, slower mass transfer. The product quality may be limited by the reactor, not the chemistry.
Energy Efficiency
Energy consumption comes from several sources. Firstly, the reaction itself may require or consume heat. The reactor requires heat to maintain its temperature and compensate for the heat loss (proportional to the external surface area). At the end of the reaction, the contents of the batch reactor are emptied and the whole process starts again; hence, all the heat input is lost. Using a large reactor means high energy requirement, and more energy to keep the temperature constant.
Catalytic reactions
In a reaction that involves heterogeneous catalyst, the catalyst is added as a slurry – a suspension of small particles. Yet, there are still several problems. During the reaction, the impeller may crush the catalyst particles. It is often challenging to separate the catalyst from the product, so the catalyst is rarely re-used. This results in low catalyst lifetime and a lot of waste.
| Subjective rating | ||||
Flexibility | ★ | ★ | ★ | ★ | ★ |
Scalability | ★ | ★ | ★ | ★ | ☆ |
Control | ★ | ★ | ★ | ☆ | ☆ |
Energy Efficiency | ★ | ★ | ★ | ☆ | ☆ |
Catalyst lifetime | ★ | ★ | ☆ | ☆ | ☆ |
Conclusion
We have given each type of reactor a subjective rating based on five parameters.
Batch reactors can be used for almost any reaction – their flexibility is excellent.
Although there some challenges, the scalability of batch reactors is good and there is a lot of scale-up experience.
Reaction control is rather limited – highly exothermic and fast reactions suffer from the limited heat and mass transfer rates. As a result, product quality suffers.
Energy efficiency is also average due to the heat required every time at start-up. Large surface and difficult heat recovery lead to energy loses.
The catalyst lifetime is moderate to poor because the (solid) catalyst separation is often challenging.
The key advantage of the batch reactors is flexibility and multipurpose operation – that is why these rectors are used in most chemical processes.
Catalyst for fuel cell applications
We have developed a monolith-based fuel cell catalyst for sustainable remote energy generation.
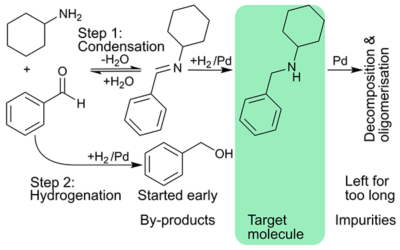
Cascading imine formation and hydrogenation
Stoli cascaded imine formation and hydrogenation; intensified process to maximise rate, and catalyst utilisation.
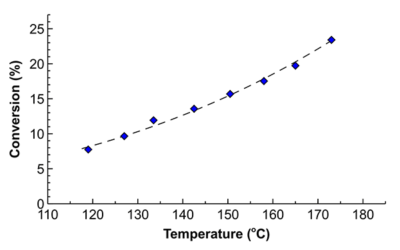
Process intensification in hydrogenation
Short residence time and high temperature – an impossible combination for batch – allowed increasing specific reaction rates 8-fold in flow with the same product quality.