Background
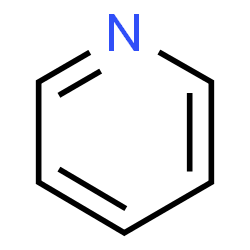
Chemisorption, as opposed to physisorption, is a strong interaction between the adsorbed molecule and the surface of the molecule of the active site. Pyridine is a base molecule that strongly interacts and chemisorbs over the acid sites of studied materials. The materials include inorganic oxides such as alumina (Al2O3), ceria or cerium oxide (CeO2), zirconia or zirconium oxide (ZrO2), silica (SiO2), titania or titanium oxide (TiO2). More complex materials and catalysts such as metal-organic frameworks (MOFs), zeolites such as ZSM-5, Mordenite, Y or beta could also be studied. The advantage of pyridine is the relatively large size – pyridine reacts with the molecules in could reach inside the material and on its external surface.
Importance of Chemisorption
Chemisorption of pyridine allows determining the number of the corresponding acid sites in the material. Such properties are essential in confirming the reproducibility and quality of the material and predict its interactions and catalytic behaviour. This includes the amount of active sites available, acidity, surface area or the ability of materials to perform after reduction/oxidation cycles.
Experimental Setup
We perform chemisorption in a bespoke system that allows carrying out both adsorption and temperature-programmed desorption (TPD) studies. The material to be analysed is placed into a quartz tube between 2 layers of quartz wool; the tube is placed into a tube furnace. Prior to the measurements, the sample may be calcined in the flow of oxygen in helium to remove any surface contaminations. The adsorption is typically performed in the temperature range of about 100 °C to prevent physical adsorption of the probe molecules over the surface. The amount of the pyridine adsorbed could be measured using a breakthrough curve technique. After the adsorption step, the sample is purged with He flow for 2h at 100°C to remove weakly adsorbed species followed by the temperature ramping at the desired heating rate, around 5-10 °C/min. The desorption products are analysed with a quadrupole mass spectrometer (QMS) determining the desorption profile.
Outcome
The pyridine chemisorption studies over the Pt/SiO2 and SiO2 materials showed that introduction of platinum metal over the support dramatically increase acidity.
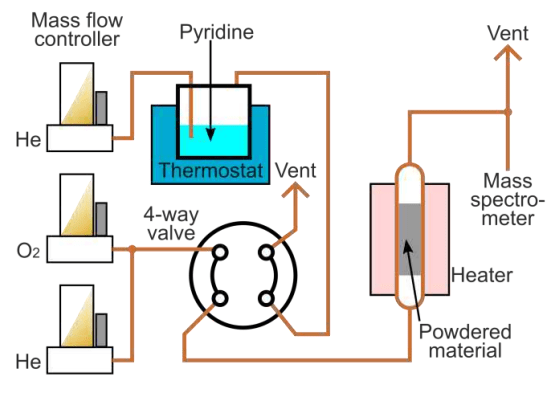
The setup used in the pyridine chemisorption study
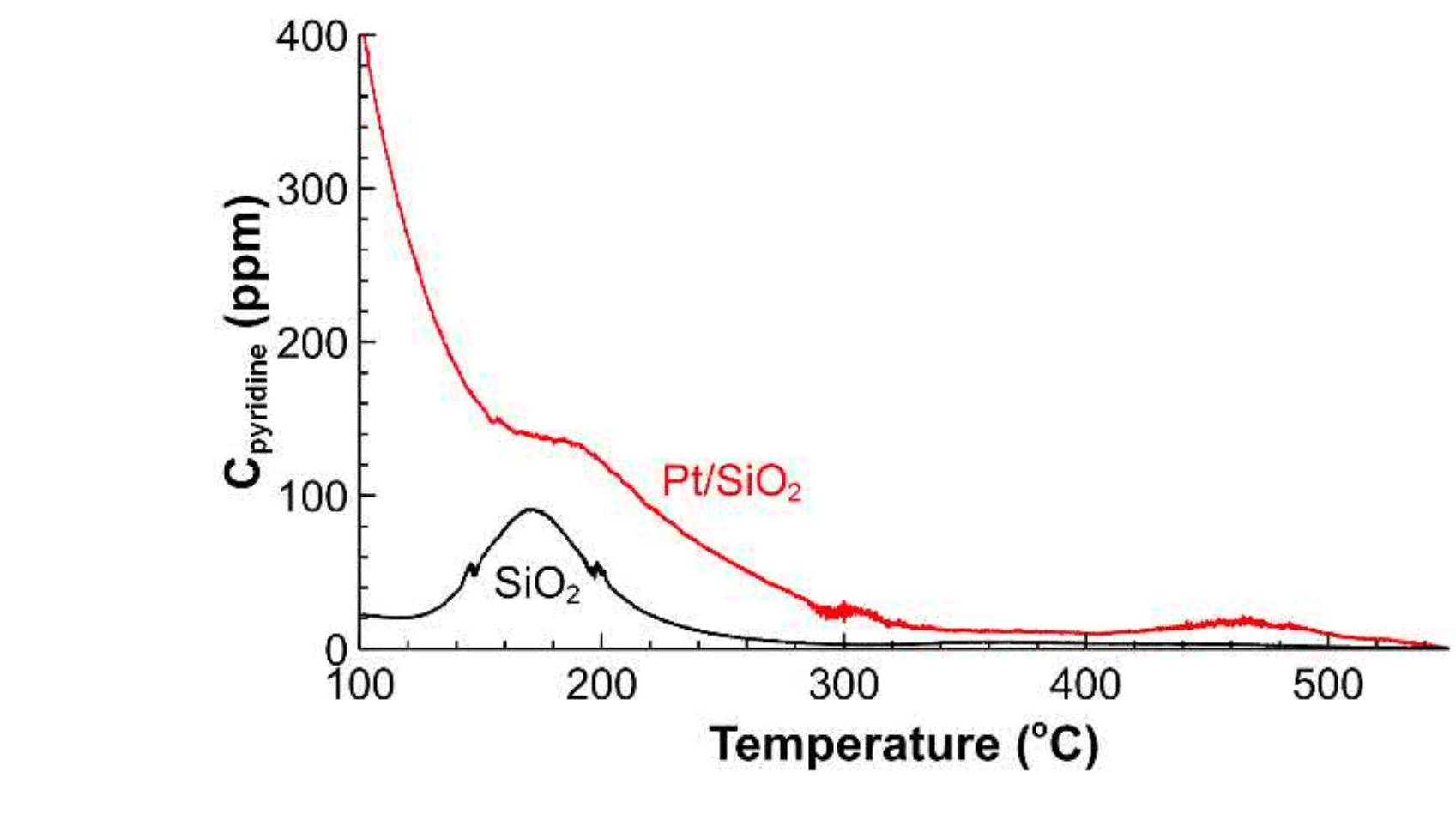
TPD profile of Pt/SiO2 catalyst and SiO2
Catalyst for fuel cell applications
We have developed a monolith-based fuel cell catalyst for sustainable remote energy generation.
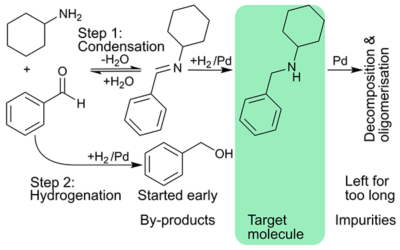
Cascading imine formation and hydrogenation
Stoli cascaded imine formation and hydrogenation; intensified process to maximise rate, and catalyst utilisation.
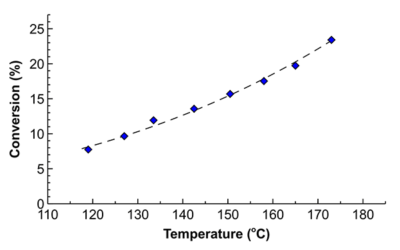
Process intensification in hydrogenation
Short residence time and high temperature – an impossible combination for batch – allowed increasing specific reaction rates 8-fold in flow with the same product quality.