The chances are you will have come into contact with at least one of these today:
the ingredients in your tooth paste, soap, plastic.
Many of us don’t give too much thought to where they come from. A lot of these things are manufactured in chemical reactors.
Daniel Lambden overviews of the tubular reactors (incorrectly namd, “plug flow”) – the simplest continuous reactors.
How do tubular reactors work?
In the simplest sense, the tubular reactor is just a pipe, a cylindrical vessel where fluids are continually flowed through, usually at a steady velocity. They might be heated or cooled through the exterior using electric heaters or a heating jacket.
In their simplest form, with single phase flow, the reactants will enter one end, react inside, and exit as products.
Tubular reactors are often used with two-phase flow such as gas and liquid. The liquid phase may flow downwards; gas – upwards. We call this counter-current flow. When the two phases are flowing in the same direction we call this co-current flow.
What type of reactions can you carry out?
Tubular reactors are versatile due to their simplicity and are often employed in processes such as the Haber-Bosch ammonia synthesis, synthetic petroleum production, or waste treatment.
The reactors may be empty, in the case of homogeneous reactions, or packed with catalyst or other solid particles for heterogeneous reactions such as in packed bed reactors.
Tubular reactors are flexible and can be made to various sizes and engineered for various pressures, temperatures and materials of construction.

Reaction control
In tubular reactors, the reaction control is dependent on the diameter of the tube. In tubes micrometers in diameter, effectively channels, heat and mass transfer rates are exceptional, however as the diameter increase heat and mass transfer rates decrease (exponentially?).
The reason is that the rate of mixing and hence mass transfer is controlled by the fluid velocity.
This is fine if you are operating at high fluid velocities and produce turbulent flow, though at low fluid velocities or with high viscosity fluids, you may have laminar flow and mixing will be limited.
In this case, mixing can be improved with the use of static mixers or using oscillating flow, but these don’t come without their own challenges.
Therefore, for microscale applications, reaction control in tubular reactors is excellent. At larger scale, reaction control and subsequently the reactor performance suffers.
Scalability
The scaling up of tubular reactors is regularly carried out, although there might be some trade-offs between reactor performance and costs.
In the wider tubes, the fluid velocity decreases. As a result, heat & mass transfer decreases.
In the longer tubes, the velocity stays constant but requires a higher pressure.
A way around this is by increasing the number of tubes. The approach is numbering up, but it proportionally increases the costs.
As a result, the trade-offs make the scalability challenging. Yet, due to the simplicity of the tubular reactors in many cases the trade-offs are an acceptable compromise.
What about Energy efficiency?
Similar to continuously stirred tank reactors, tubular reactors are quite energy efficient because it is continuous and avoids stopping and starting observed in batch processes.
The reaction and process heat could be recovered from the product stream improving the overall process efficiency.
Yet, often a high fluid velocity is required to maintain heat & mass transfer and more energy is required to push the fluids through.
Catalyst lifetime (for heterogeneous catalysis)
Catalyst lifetime in continuous tubular reactors can vary depending on the process.
There are several key sources of catalyst deactivation.
Mainly deactivation comes from the intrinsic chemistry of the process – for example through reaction with some minor impurities or by-products.
Deactivation may also come from the deviations in the process conditions. For example, the limited heat removal may result in hot spots and rapid localised catalyst deactivation.
Having the catalyst fixed (or static) enables easy product separation and avoids deactivation due to mechanical wear and tear (attrition), In some cases the catalyst may remain quite active for months or years.
| Subjective rating | |||||
| Flexibility | ★ | ★ | ☆ | ☆ | ☆ |
| Scalability | ★ | ★ | ★ | ☆ | ☆ |
| Control | ★ | ★ | ★ | ☆ | ☆ |
| Energy Efficiency | ★ | ★ | ★ | ★ | ☆ |
| Catalyst lifetime | ★ | ★ | ★ | ☆ | ☆ |
Conclusion
The tubular reactors are simple and easily maintained. They provide an efficient use of reactor volume.
On the downside, the tubular reactors don’t offer great reaction control. Hotspots may occur with exothermic reactions and mixing is dependent on fluid velocities.
Despite this, the tubular reactor continues to be a mainstay of the petrochemical industry due to its simplicity, flexibility and comparatively low space requirements.
Catalyst for fuel cell applications
We have developed a monolith-based fuel cell catalyst for sustainable remote energy generation.
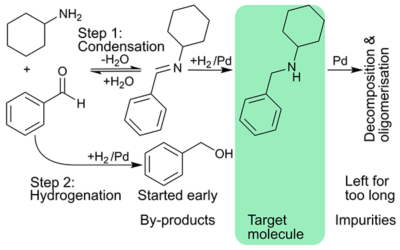
Cascading imine formation and hydrogenation
Stoli cascaded imine formation and hydrogenation; intensified process to maximise rate, and catalyst utilisation.
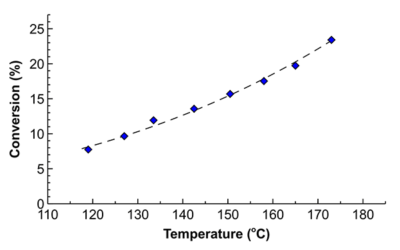
Process intensification in hydrogenation
Short residence time and high temperature – an impossible combination for batch – allowed increasing specific reaction rates 8-fold in flow with the same product quality.