Most chemical reactions release heat and many require accurate thermal control to maintain an optimum temperature. It is not only a question of safety and preventing reaction runaway, it is often a question of product yield and quality.
The Michael addition of amines to α,β-unsaturated carbonyls, for example, results in reasonable yields of 85%. However, this process requires long reaction times of up to 24 hours. This is not due to intrinsic kinetics, but because of high reaction exotherm. Managing the generated heat requires the slow addition of reactants.
The addition of dimethyl amine (40 wt% in water) to an α,β-unsaturated carbonyl dropwise in a 500mL round bottom flask proceeds on the order of 16 h. Löwe et al. [1] performed the same reaction in a microreactor (stainless steel platelets, 0.015 mL), reducing the batch time of 16 h to a residence time of 7.2 minutes with a yield of >95%. This was due to the extremely high heat transfer of the microreactor, measured at 4000 W/m²/K. This is in comparison to the 500 mL batch reactor with an overall heat transfer coefficient of 185 W/m²/K [2]. Yet, scaling microreactors to kilos and tonnes is difficult. Increasing channel diameters dramatically reduces heat transfer and reactor performance. Putting a higher flow rate through the reactor requires huge pressure.
Our novel Scalable Agitated Baffle Reactor (SABRe) provides rapid heat transfer, simplicity, versatility and predictability. We have calculated the heat transfer improvement comparing with the same ~ 130 mL reactor considering the worst-case scenario of the same heat-transfer performance. So, can SABRe still beat batch?
Outcome
The calculation of the worst-case scenario shows that in managing such exothermic reactions the throughput of SABRe is 1.75 times higher than that of the batch, not taking into account reduced downtime, safety, labour and other costs.
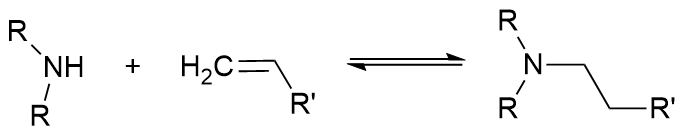
Addition of secondary amines to unsaturated carbonyl compounds
References:
[1] H. Löwe, V. Hessel, P. Löp, S. Hubbard, Org. Process Res. Dev. 10 (2006) 1144–1152.
[2] M. Johnson, P.J. Heggs, T. Mahmud, Org. Process Res. Dev. 20 (2016) 204–214.
| Batch | SABRe | |
| Diameter (cm) | 6 | 2.6 |
| Height (cm) | 5 | 25 |
| Volume (mL) | 141 | 133 |
| Wall area (cm²) | 123 | 215 |
Heat dissipation | ||
| Overall heat transfer coefficient (W/m²/K) | 500 | |
| Log mean temperature difference with heat exchange fluid (C) | 30 | |
| Heat dissipated (W) | 184 | 322 |
Heat generation | ||
| Reaction enthalpy (kJ/mol) | 220 | |
| Flow rate (mmol/min) | 50.2 | 87.9 |
| Reaction heat (W) | 184 | 322 |
| Flow rate increase | 1.75 X | |
Catalyst for fuel cell applications
We have developed a monolith-based fuel cell catalyst for sustainable remote energy generation.
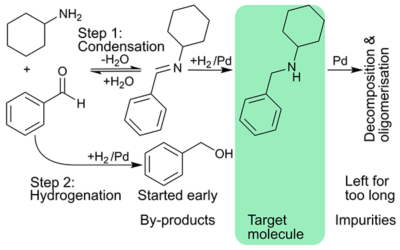
Cascading imine formation and hydrogenation
Stoli cascaded imine formation and hydrogenation; intensified process to maximise rate, and catalyst utilisation.
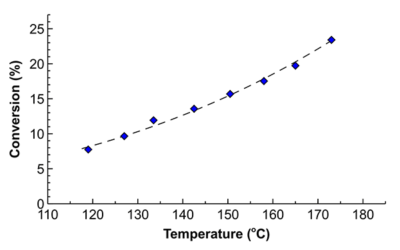
Process intensification in hydrogenation
Short residence time and high temperature – an impossible combination for batch – allowed increasing specific reaction rates 8-fold in flow with the same product quality.