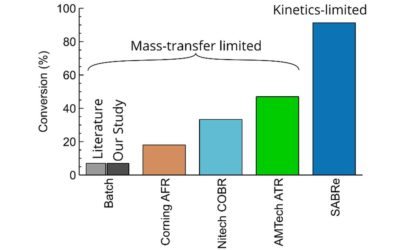
Reactions often involve gases and liquids, from conventional chemistry to complex biological processes. The applications are broad and can be found in research labs from gram scales, to kiloton production facilities.
When assessing or designing a process, it is vital to have fundamental knowledge about reactor performance that is independent of the process. With this information, the limiting stages in the process can be calculated and the possibilities for scale-up can be evaluated.
The gas-liquid mass transfer coefficient is a key parameter, and one of the most widely used performance indicators in chemical engineering of multiphase reactions.
Download pdf
Case Study # 03, rev 6,
17 Aug 2021, By Dr Joe Socci
What is the mass-transfer coefficient?
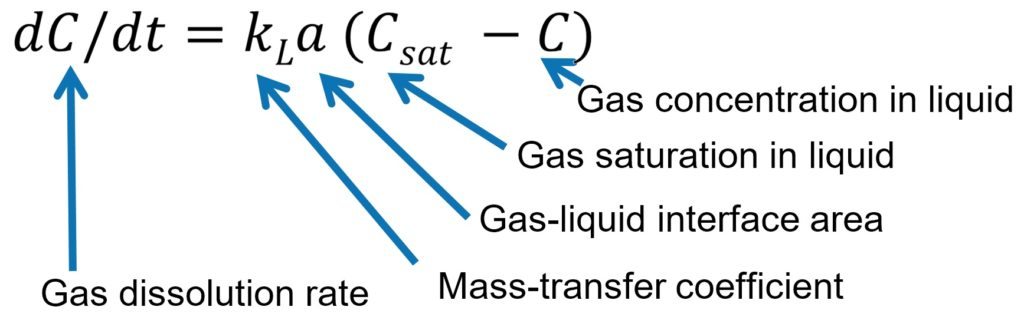
The rate of gas dissolution in liquid often limits and determines the rate of a gas-liquid process.
The gas dissolution rate is proportional to the gas concentration difference between saturation and current concentration. The proportionality coefficient, kLa is the mass-transfer coefficient.
In practice, the gas saturation is fixed as it depends on temperature and pressure or the existing gas concentration (often determined by the process requirements).
You could optimise a reactor and its operating parameters to maximise kLa; therefore, maximising the performance of the process. For example, in mass-transfer limited reactions, doubling the kLa value of the reactor would double the product throughput. The higher the value the better.
Gas-liquid processes:
- Catalytic hydrogenation
- Gas scrubbing
- Catalytic oxidation
- Enzymatic oxidation
- Aerobic digestion
- Fermentation
Typical values in a batch reactor
In batch reactors, typical kLa values are between about 5 and 50h-1 depending mainly on the mixing power and speed. Small reactors could introduce large specific mixing power, split gas bubbles and provide rapid circulation, but they have limited contact gas-liquid contact time. Larger reactors have a lower surface area to volume ratio. Yet, maintaining constant mixing power per litre of volume allows to maintain constant kLa with scale-up.[4]
Small batch reactors with volumes in the region of 100 mL could reach values of ~1000 h-1, with impeller mixing almost a factor of 100 times more efficient than magnetic stirring.[5] Microreactors, similar to smaller batch reactors, could reach impressive kLa values of 10,000 h-1 at a sub-millilitre volume but rapidly lose their performance with scale.
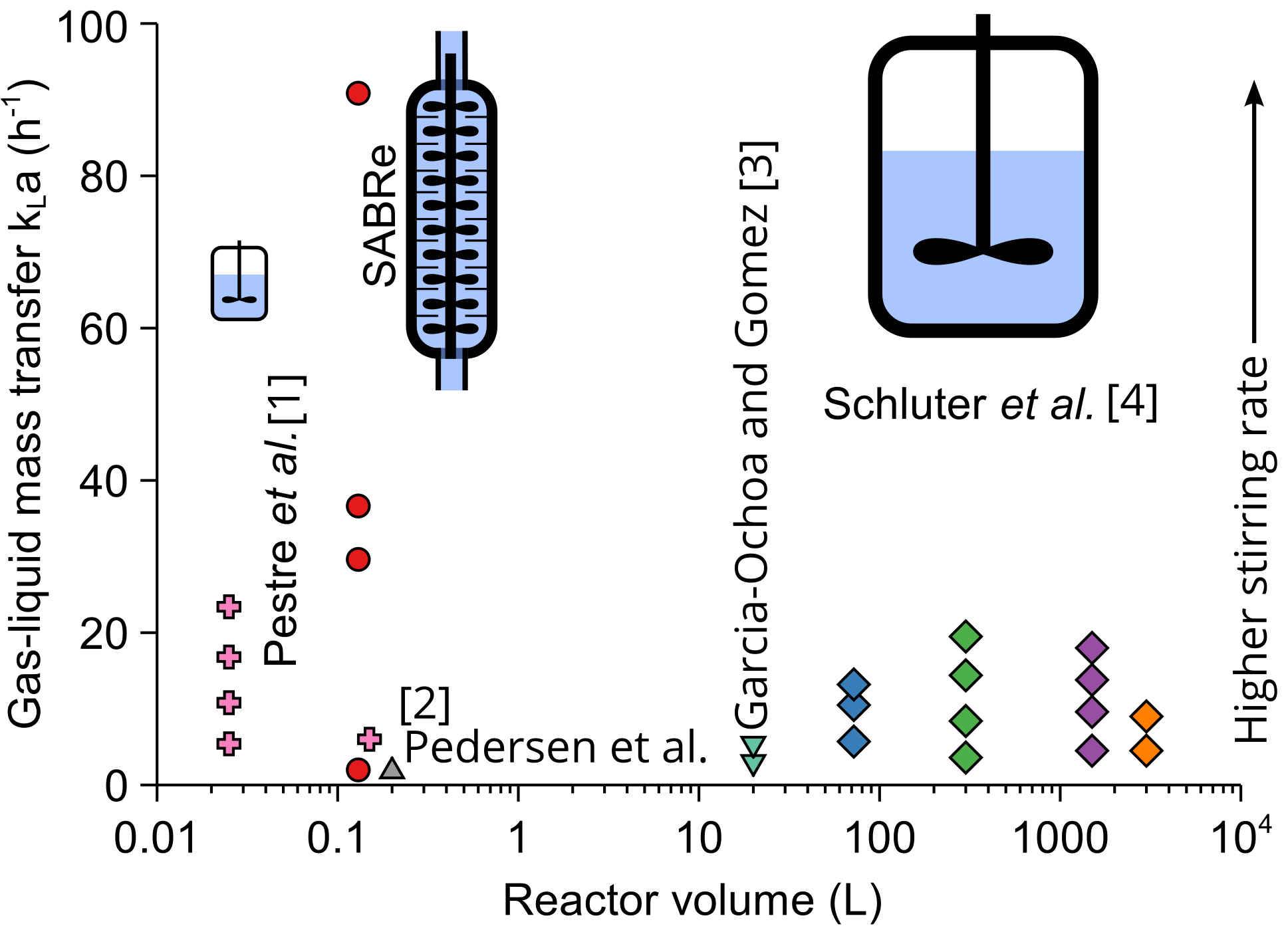
Predictions and correlations
There has been extensive research into correlations for the prediction of the gas-liquid mass transfer coefficient in agitated chemical reactors. However, due to the complexity of gas-liquid systems and sizeable differences in reactor geometries, no single correlation completely represents all of the data for gas-liquid mass transfer coefficient in the literature. Nevertheless, it is possible to predict the gas-liquid mass transfer coefficient in multiple impeller reactors over a range of scales with high accuracy. [6]
Typical kLa correlation:
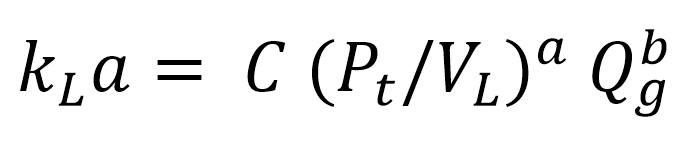
kLa = volumetric liquid-side mass transfer coefficient (s-1)
Pt = total impeller power consumption (W)
VL = liquid volume in vessel (m3)
Qg = superficial gas velocity (m s-1)
C = constant
a, b = exponents
The gas-liquid mass transfer coefficient (kLa) of the Scalable Agitated Baffle Reactor (SABRe) was measured at different gas volumetric flow rates and a range of impeller speeds to determine their effect on the mass transfer in an air/water system.
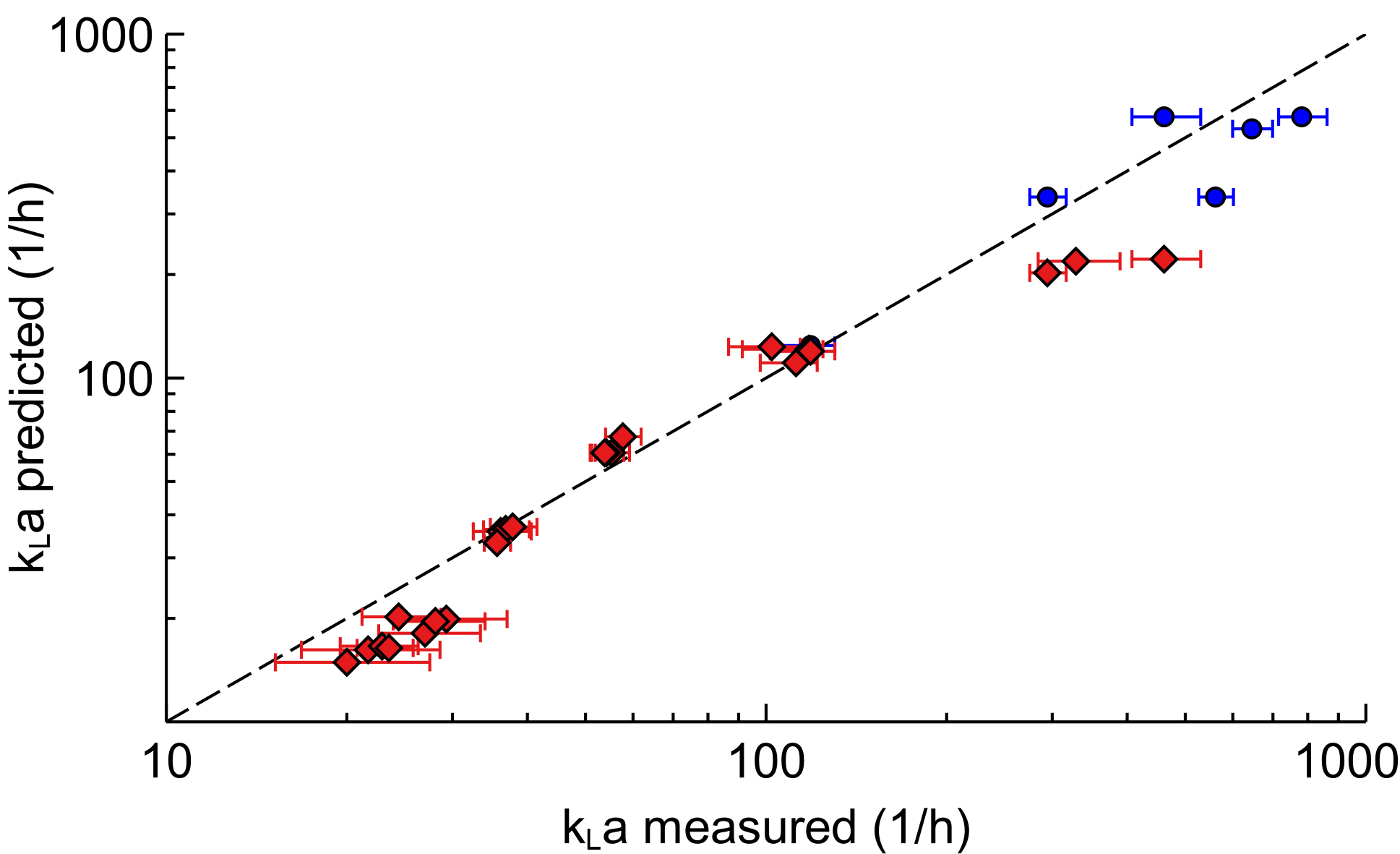
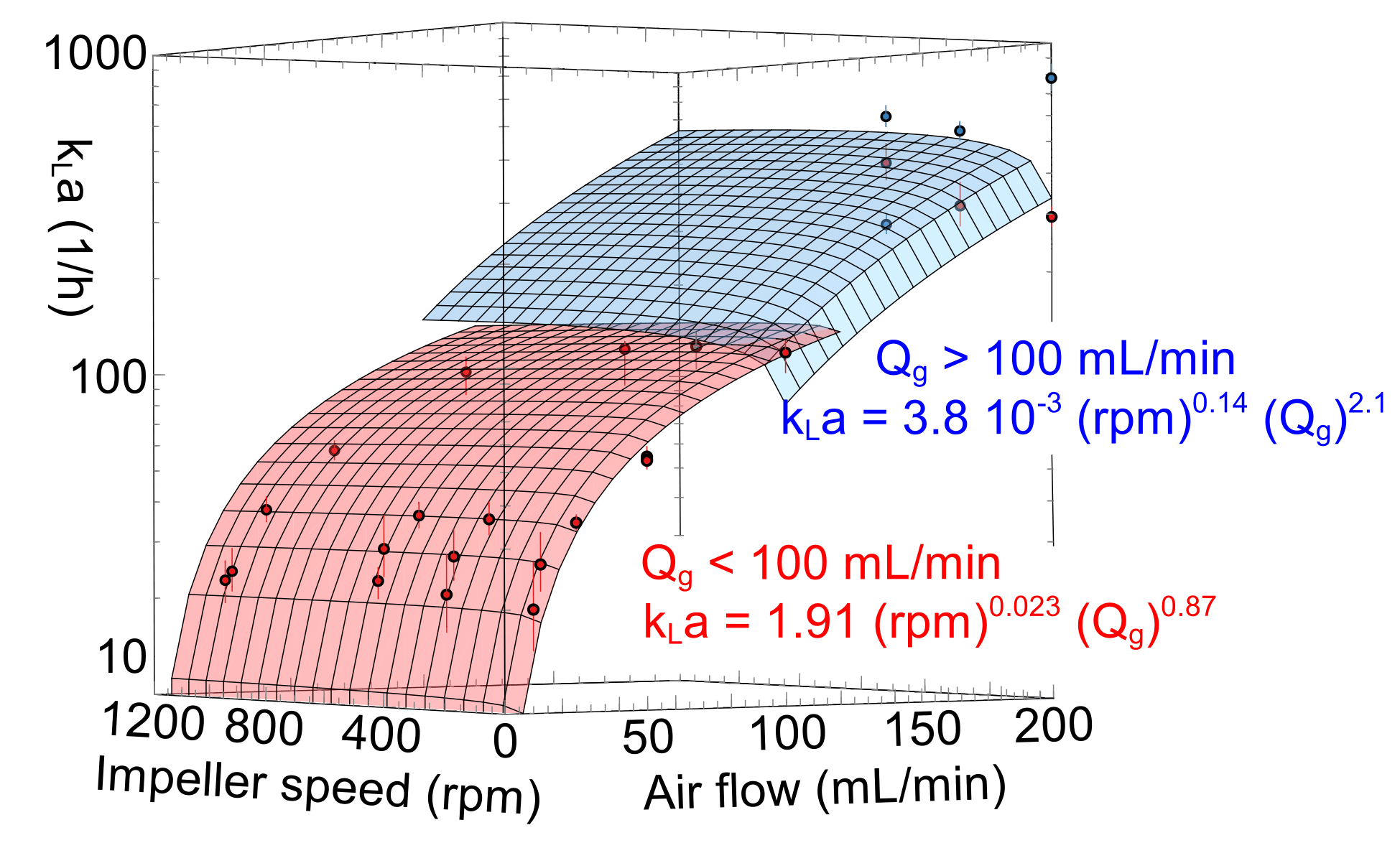
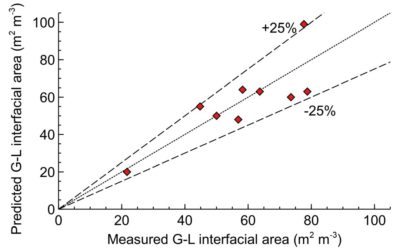
As shown, there is excellent fit between predicted and experimental mass transfer performance of SABRe, showing a high degree of reliability. Using these correlations allows accurate prediction of the behaviour of the reactor in gas-liquid reactions such as catalytic hydrogenations, gas scrubbing and fermentation. They can also be used to predict reactor performance as the scale of the process is increased – this allows informed decisions on the economics, laboratory footprint and heating or cooling required.
The SABRe system provides rapid gas-liquid mass transfer and predictable scalability beyond 5,000L for high process throughput.
References [1] N. Pestre, P. Fongarland, C. De Bellefon, Ind. Eng. Chem. Res. 43 (2004) 924–927; [2] A. Toftgaard Pedersen, T.M. de Carvalho, E. Sutherland et al., Biotechnol. Bioeng. 114 (2017) 1222–1230; [3] F. García-Ochoa, E. Gómez, Biochem. Eng. J. 1 (1998) 1–10; [4] V. Schlüter, W.D. Deckwer, Chem. Eng. Sci. 47 (1992) 2357–2362; [5] V. Meille, N. Pestre, P. Fongarland, C. De Bellefon, Ind. Eng. Chem. Res., (2004) 924–927; [6] J. Markopoulos, C. Christofi, I. Katsinaris, Chem. Eng. Tech., (2007) 829–834
The SABRe system (available in steel, Hastelloy or glass) is suitable for a wide range of chemical applications. Combining simplicity with superb reaction control, SABRe is the best choice for simple, safe and cost effective chemistry.
What can the SABRe do for you today? Get in touch and arrange a trial.
Other SABRe case studies:
Improvement of enzymatic oxidation in the continuous Scalable Agitated Baffle Reactor (SABRe) system
Case study on enzymatic oxidation by Prof John Woodley
Steven’s oxidation with Vapourtec
1.4 kg/day multiphase oxidation obtained integrating SABRe system with Vapourtec’s R-Series
Consistent oil-in-water emulsions in continuous flow
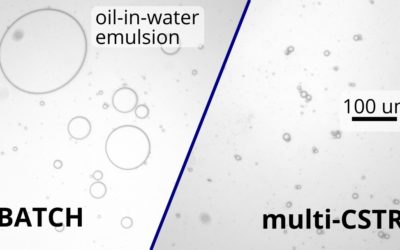
Using a continuous multi-CSTR system allowed us to make droplets 2.5 times more uniform compared to a batch reactor
How to calculate heat transfer in continuous flow applications
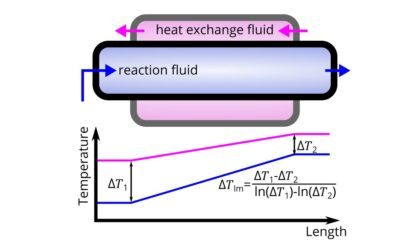
Continuous flow (such as micro-reactors) are superior for exothermic reactions. How do you compute the thermal performance of a reactor?
Maximising interfacial gas-liquid area with Scalable Agitated Baffle Reactor (SABRe)
How the SABRE system provides large gas-liquid area to maximise the reaction throughput and selectivity.
Comparison of continuous reactors in enzymatic esterification
We showed superior performance of SABRe in the enzymatic (liquid-liquid) esterification.